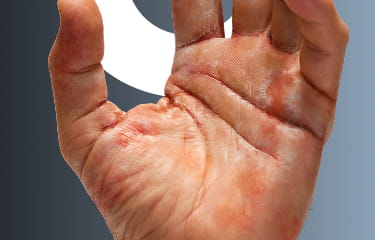
The first and only treatment for adults with moderate-to-severe Chronic Hand Eczema (CHE)1,2

Actor portrayal.
Significant
clearance your
patients can see and
feel1
Clinical trial
photos
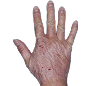
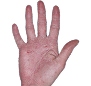
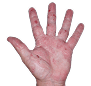
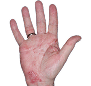
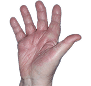
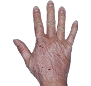

Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
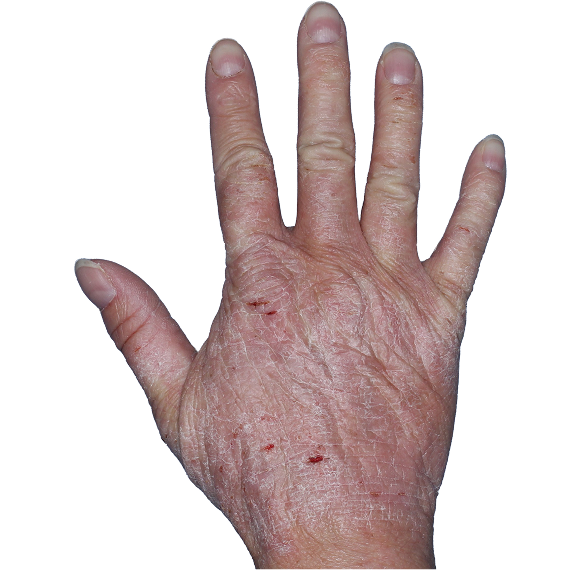
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
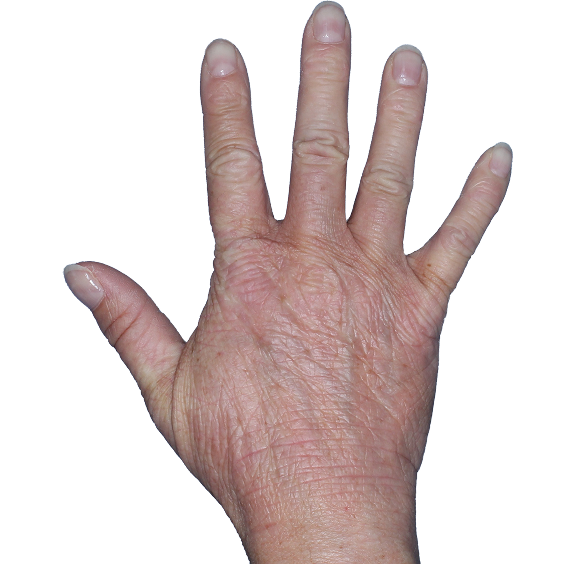
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
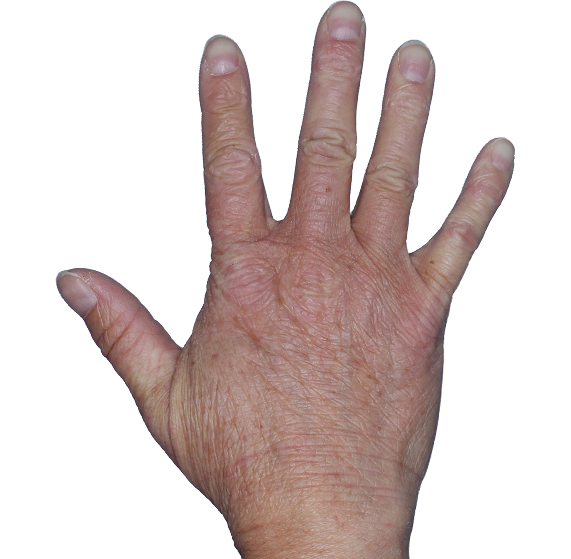
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
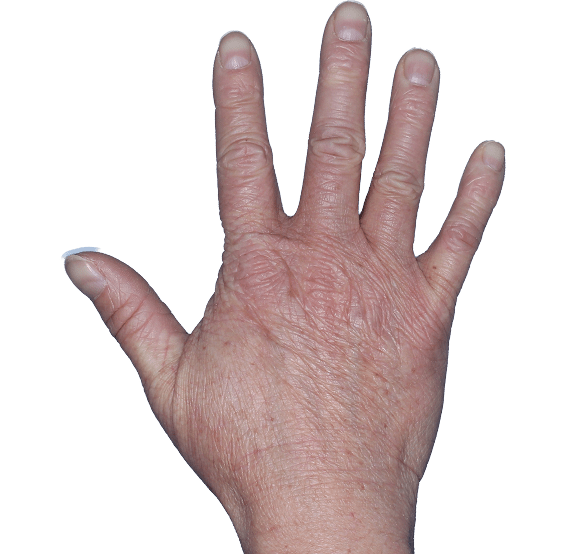
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
Case 1
- Scaling
- Cracks
- Fissures
ANZUPGO clinical trial patient.
Individual results may vary.
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
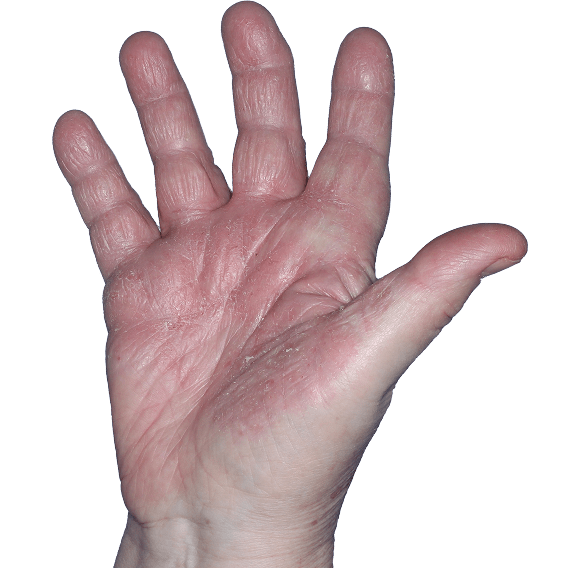
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.

Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
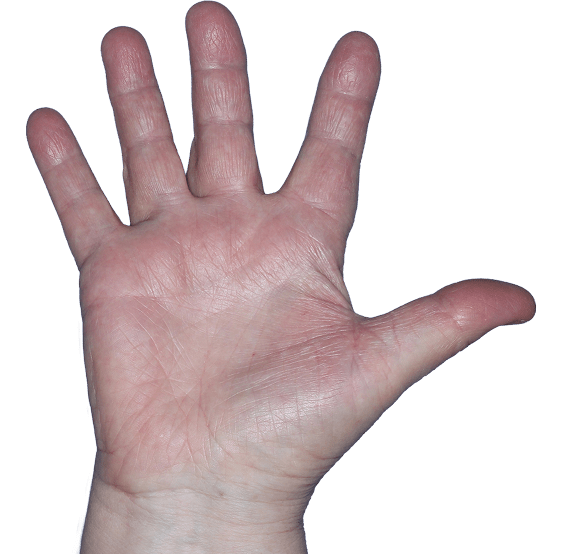
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
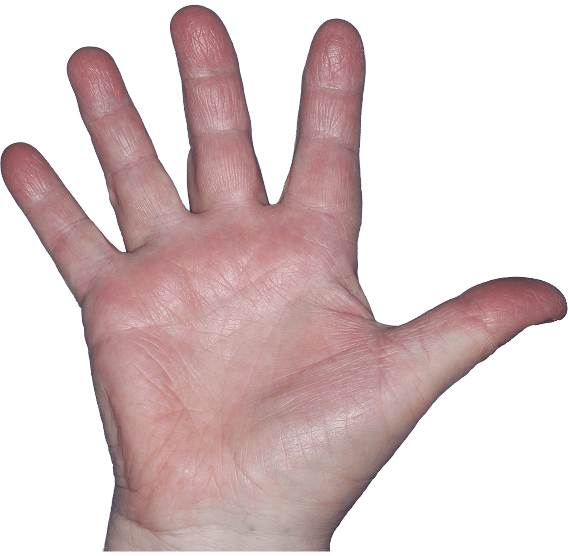
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 2
- Scaling
- Erythema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
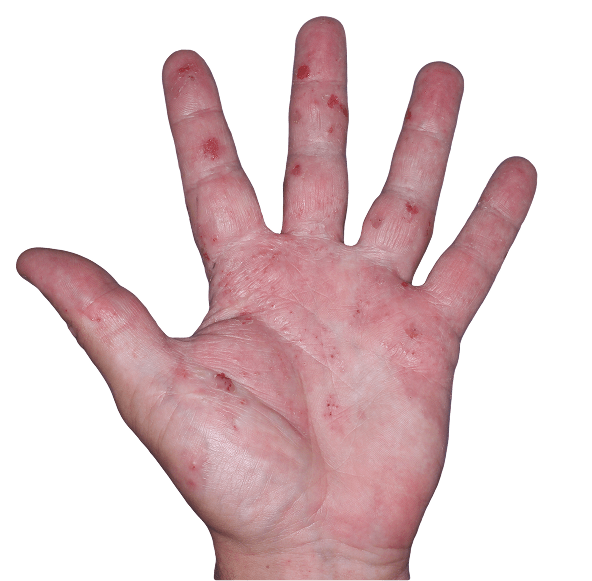
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
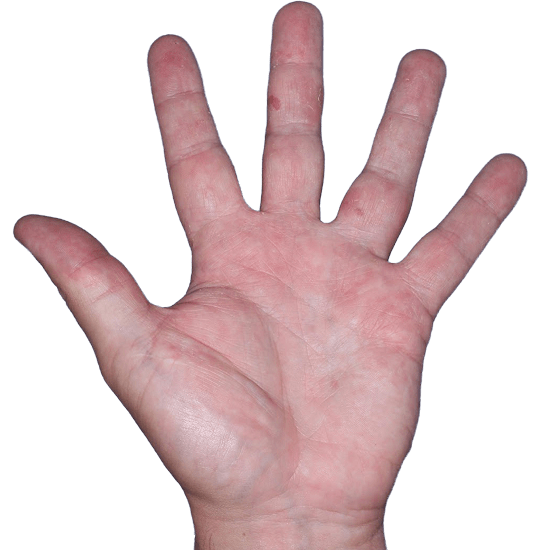
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
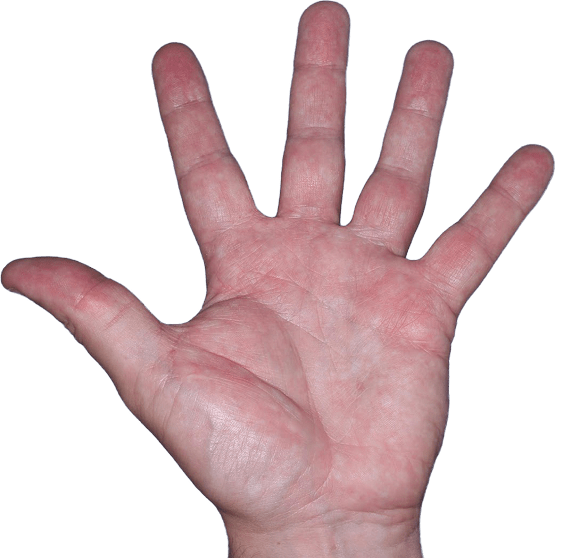
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
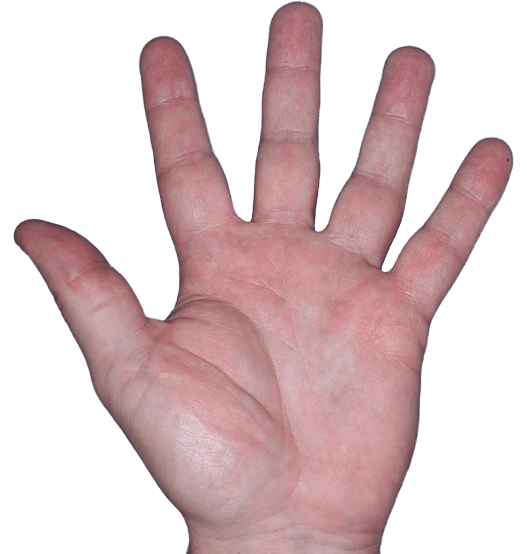
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 3
- Vesicles
- Erythema
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
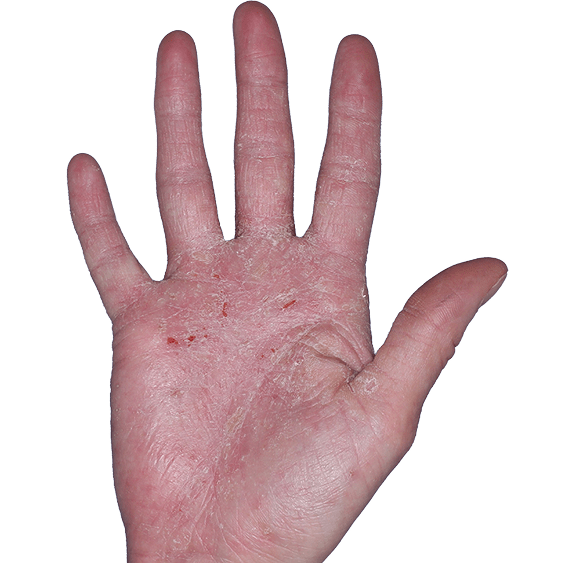
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
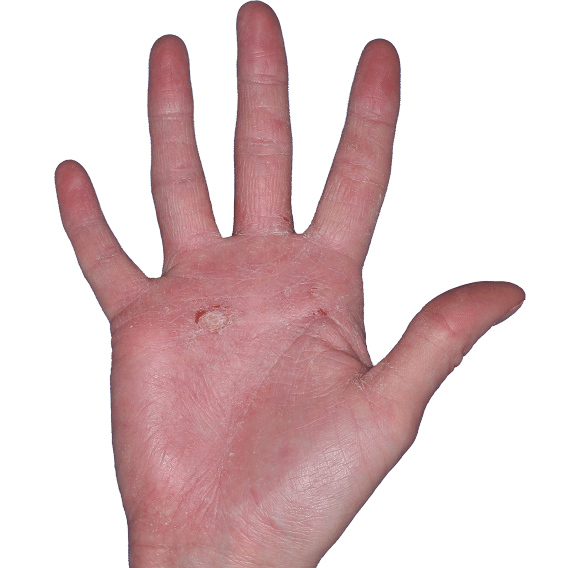
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
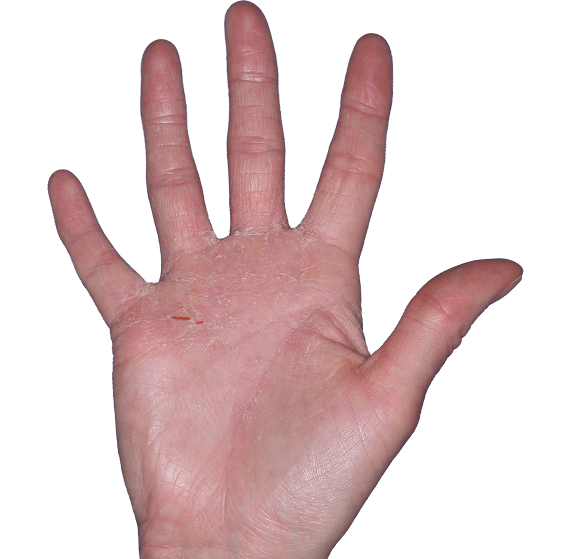
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
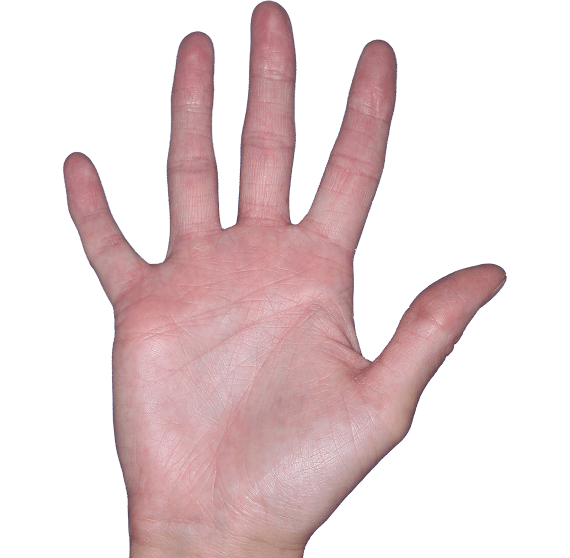
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 4
- Erythema
- Scaling
- Erosions
ANZUPGO clinical trial patient.
Individual results may vary.
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
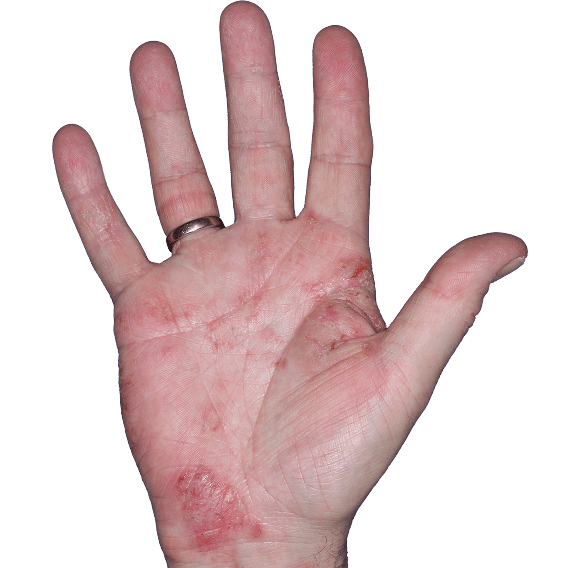
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
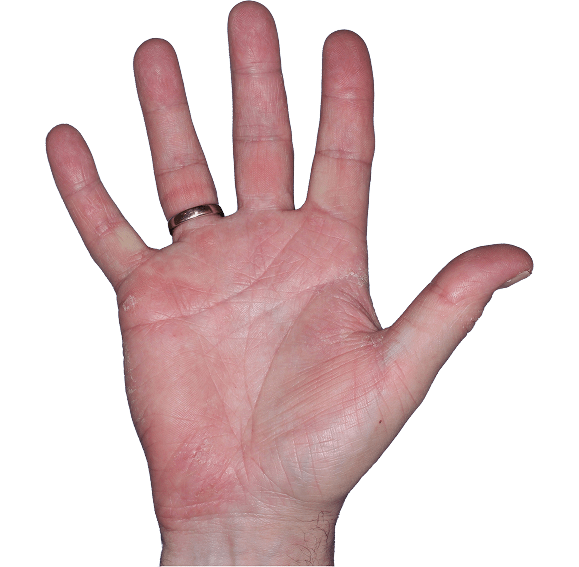
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
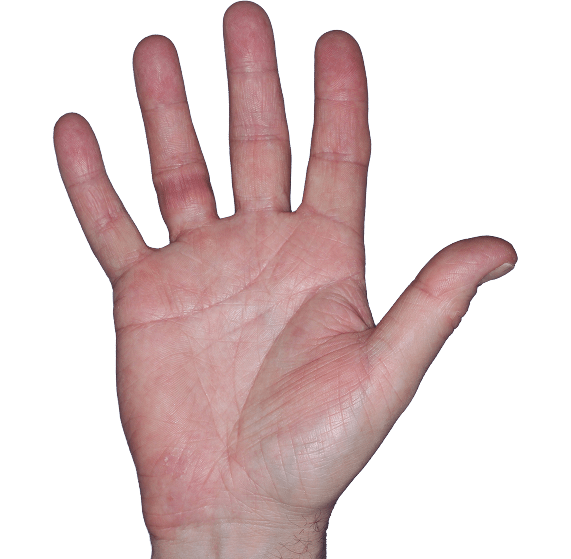
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
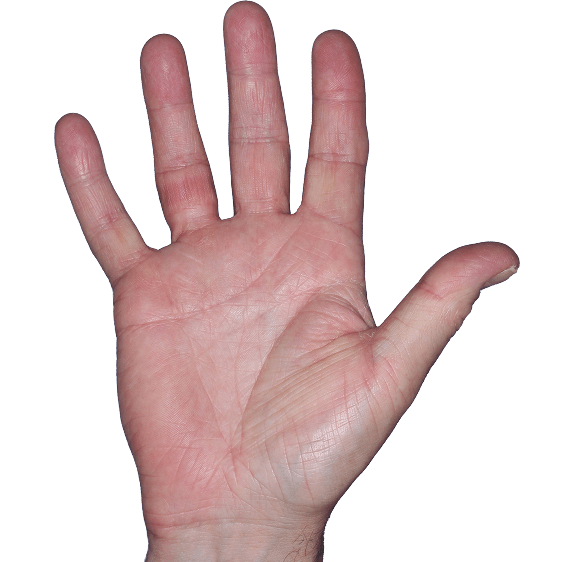
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
Case 5
- Erythema
- Scaling
- Fissure
ANZUPGO clinical trial patient.
Individual results may vary.
Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.
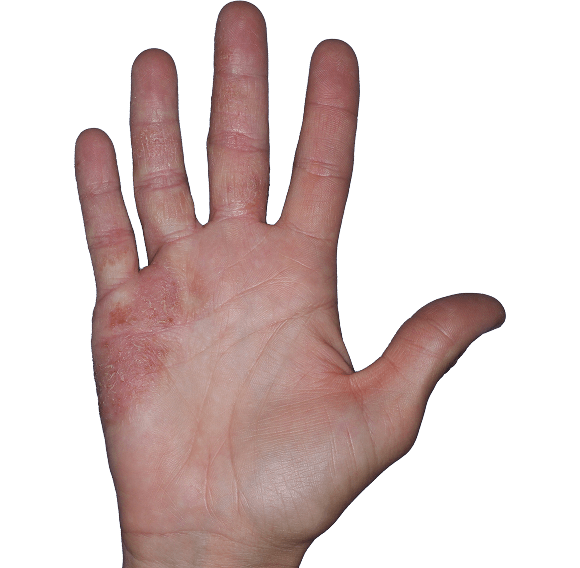
Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.

Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.

Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.

Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.
Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.
Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.
Case 6
- Erythema
- Scaling
ANZUPGO clinical trial patient.
Individual results may vary.
Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.
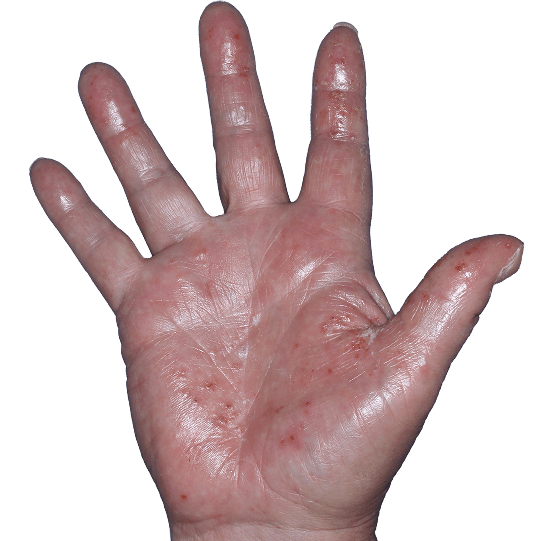
Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.

Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.

Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.

Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.
Case 7
- Vesicles
- Erythema
- Edema
ANZUPGO clinical trial patient.
Individual results may vary.
HESD=Hand Eczema Symptom Diary, a self-reported 11-point rating scale designed to measure the 6 CHE signs and symptoms3;
IGA-CHE=Investigator's Global Assessment for Chronic Hand Eczema (a 5-point assessment scale: 4, severe; 3, moderate; 2, mild; 1, almost clear; 0, clear).1,4
Access, support, resources, & more
Get the most out of ANZUPGO for you and your patients
Specifically formulated for hands
ANZUPGO was formulated specifically to take on CHE